Have you ever wondered what gives metals like aluminum their unique chemical behavior? The answer lies in something called ionic charge. When you see the term “charge of aluminum,” it refers to the electrical charge an aluminum atom carries after it gains or loses electrons during chemical reactions. This simple concept is at the heart of how aluminum interacts with other elements—and why it’s so widely used in everything from soda cans to airplanes.
In chemistry, an element’s ionic charge tells us whether its atom has more protons (positively charged) or electrons (negatively charged). A neutral atom has equal numbers of both, but when it reacts, it can lose or gain electrons, resulting in a net charge. This new charged particle is called an ion. For example, when you ask, what is the charge of an aluminum ion, you’re really asking: does aluminum lose or gain electrons, and how many?
Aluminum stands out because it almost always forms an ion with a +3 charge, written as Al³⁺. This means that a neutral aluminum atom loses three electrons, becoming a positively charged ion. If you were to predict the charge that an aluminum ion would have, the answer is nearly always +3. This consistency is due to aluminum’s atomic structure, which makes it energetically favorable for the atom to shed three electrons and achieve a more stable, lower-energy state.
Aluminum's predictable +3 charge is the foundation of its chemical behavior and utility.
Throughout this article, you’ll discover not only what is the charge of aluminum, but also why this charge is so reliable and how it shapes the ways we use aluminum every day. From its atomic roots to its real-world applications, understanding the aluminum charge unlocks the science behind one of the world’s most essential metals. Ready to explore how and why aluminum always becomes Al³⁺? Let’s dive in.
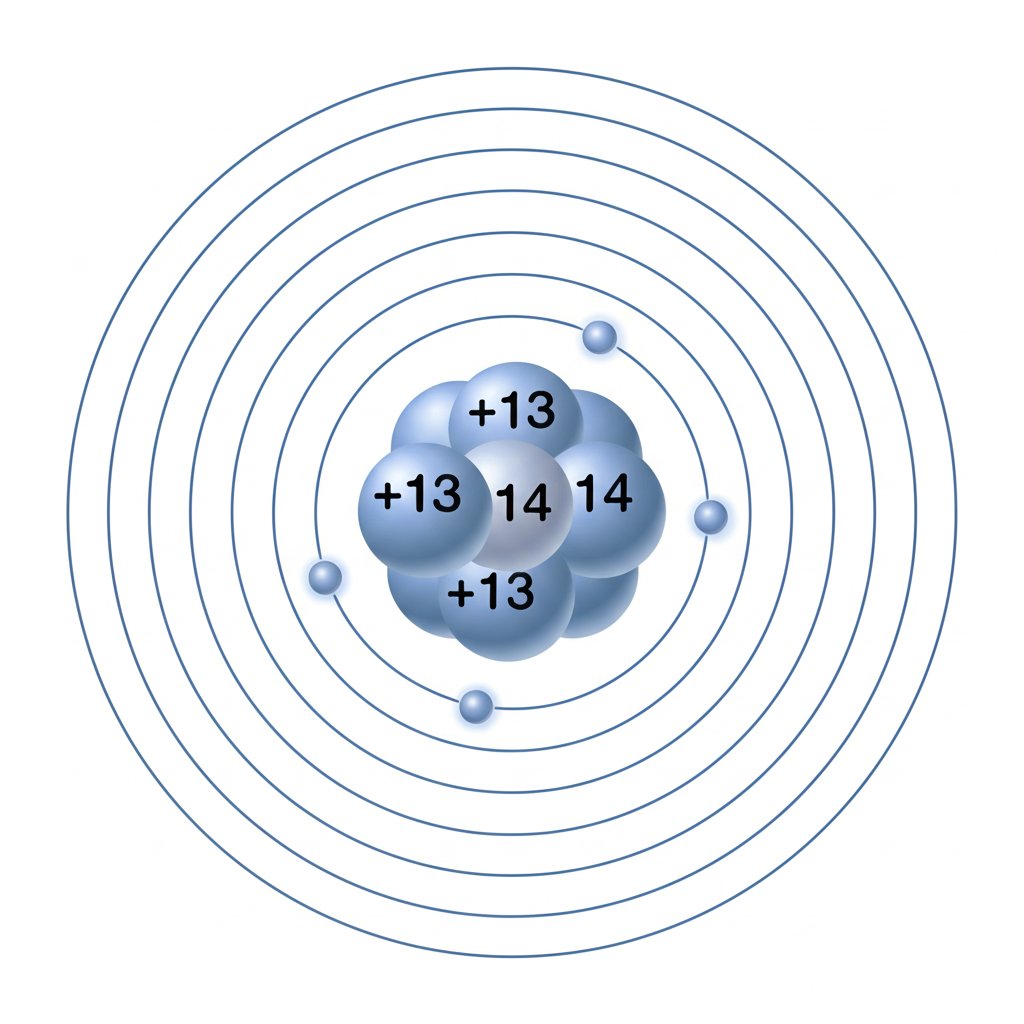
When you first look at the aluminum periodic table entry, you’ll notice aluminum sits in Group 13 and Period 3. This position isn’t just a label—it tells you a lot about how aluminum behaves. Group 13 elements all have similar outer electron arrangements, which is key to understanding why aluminum forms the charge it does. The aluminum atomic number is 13, meaning every neutral atom of aluminum contains exactly 13 protons in its nucleus. That’s the fingerprint of aluminum—no other element has this combination.
But what about the other subatomic particles? To fully grasp the charge of aluminum, it’s helpful to break down its atomic structure. Here’s how a neutral aluminum atom is built:
| Particle | Count | Charge |
|---|---|---|
| Proton | 13 | +1 |
| Electron | 13 | -1 |
| Neutron | 14 | 0 |
This table sums up the aluminum protons neutrons electrons count for a neutral atom. The 13 protons (positive charges) are balanced by 13 electrons (negative charges), making the atom electrically neutral. The 14 neutrons have no charge but add mass and stability to the nucleus. If you’re wondering how many neutrons does aluminum have, the most common isotope—aluminum-27—has 14 neutrons (27 total particles minus 13 protons).
Understanding this atomic makeup is crucial. The atomic structure for aluminum determines how—and why—aluminum atoms interact with other elements. The number of electrons in the outer shell (valence electrons) will soon explain why aluminum so predictably forms a +3 ion.
For a deeper dive into how these atomic details connect to aluminum’s behavior in technology and industry, check out this related blog: Aluminum Charge Explained: From Atomic Structure to Modern Tech.
Now that you know exactly how many protons, neutrons, and electrons are in a neutral aluminum atom, you’re ready to see how the arrangement of those electrons—especially in the outer shell—sets the stage for aluminum’s predictable charge. Next, we’ll explore the role of valence electrons and how they drive aluminum’s chemistry.
When you picture an atom, imagine layers of electrons circling the nucleus—like planets around a sun. The electrons in the outermost layer are called valence electrons. These are the electrons that get involved when atoms bond with each other. Why are they so important? Because valence electrons determine how an element reacts chemically, what kinds of bonds it forms, and ultimately, the charge it will carry as an ion.
Sounds complex? Think of valence electrons as the “handshake” part of the atom—what it uses to connect with other atoms and make compounds.
So, how many valence electrons does aluminum have? To answer this, let’s look at the aluminum electron configuration. Aluminum’s atomic number is 13, so it has 13 electrons arranged as follows:
Written in condensed form, the electron configuration for aluminum is [Ne] 3s23p1 (source). The electrons in the 3s and 3p orbitals—two in 3s and one in 3p—are the outermost electrons, making up the number of valence electrons in aluminum.
In summary:
If you’ve ever wondered about the number of valence electrons in al or how many valence electrons does al have, the answer is always three. This trio of electrons is why aluminum so consistently forms a +3 ion, as it easily sheds all its valence electrons to achieve the stable electron configuration of neon—a noble gas.
Understanding al valence electrons is the key to predicting not only the charge of aluminum but also its role in chemical reactions and compounds. Next, we’ll see exactly how losing these three electrons transforms a neutral aluminum atom into a stable, positively charged ion.
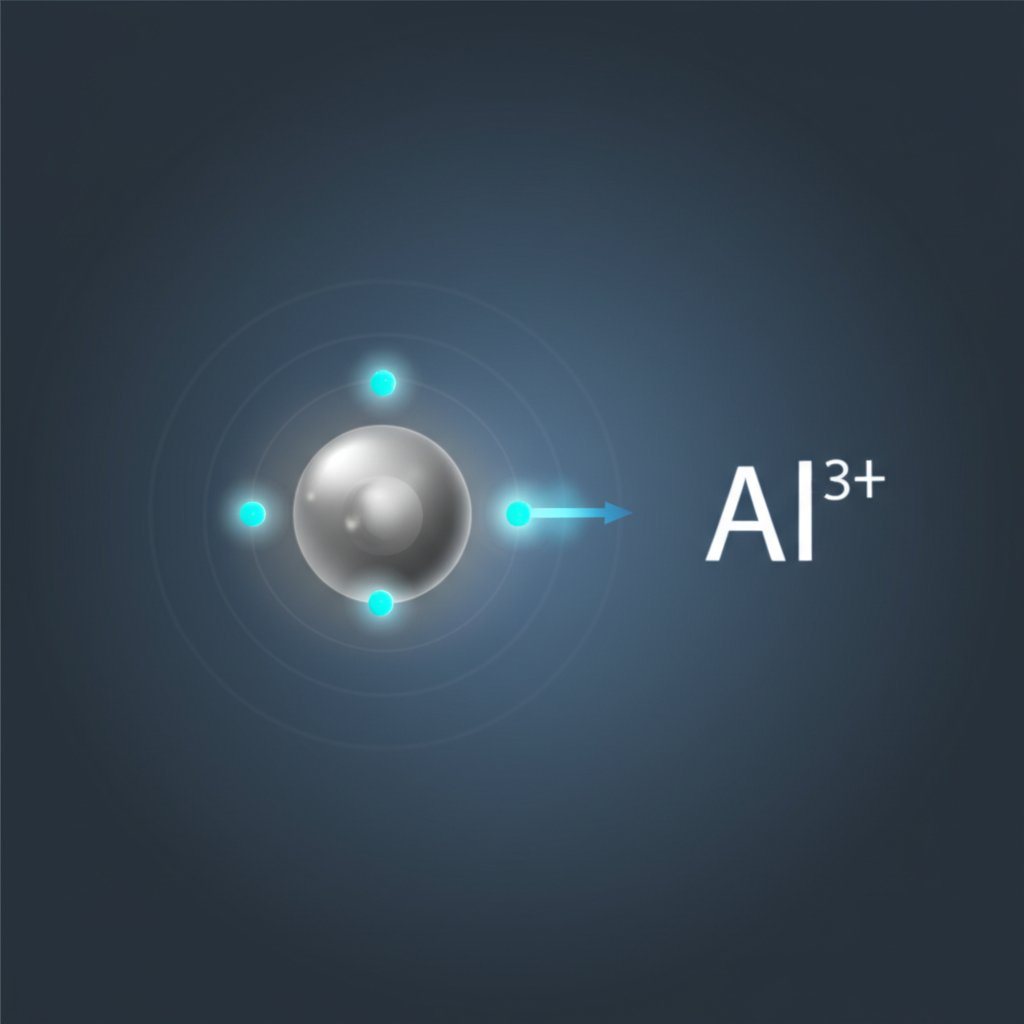
Imagine you’re holding a neutral aluminum atom. It has 13 protons (positive charges) and 13 electrons (negative charges), so the overall charge is zero. But what happens when aluminum reacts with other elements? Why does it always seem to end up with a +3 charge? The answer lies in the process of ion formation—a journey that turns a neutral atom into a stable ion by losing electrons.
When you ask, how does an atom of aluminum become an ion, the answer is simple: it loses electrons. Specifically, aluminum sheds its three outermost (valence) electrons. This process isn’t random; it’s driven by aluminum’s quest for stability. By losing these three electrons, aluminum achieves the same electron configuration as neon, a noble gas known for its stability. This is a classic case of aluminum loss or gain of electrons—but in aluminum’s case, it’s always a loss.
So, what does this transformation look like at the atomic level? Let’s break it down step by step, so you can see exactly how the cation of aluminum is formed:
This process is not unique to aluminum, but what makes it special is that it always loses exactly three electrons under normal conditions, giving it a consistent al ion charge of +3. The new ion is much more stable than the neutral atom because it has a full outer shell—just like the noble gas neon.
Let’s visualize the change:
Why does this matter? Because this transformation underpins aluminum’s reactivity, the way it forms compounds, and its behavior in everything from kitchen foil to airplane parts. If you’re ever asked, how many electrons does aluminum have after ionization, the answer is 10. That’s the signature of the al 3 ion.
By understanding this step-by-step process, you can confidently explain how the charge of aluminum comes to be—and why it’s so predictable. Next, we’ll explore why aluminum always stops at +3, and why it never forms +1 or +2 ions in nature.
Ever wondered why aluminum forms an ion with a charge of +3—and not +1 or +2 like some other metals? The answer lies in the energy it takes to remove electrons from an atom, a concept called ionization energy. Each time you pluck an electron away from an atom, you have to overcome the pull of the nucleus. But not all electrons are held equally tight. The outermost ones—the valence electrons—are easier to remove, while those closer to the nucleus are much harder to take away.
Let’s take a closer look at the al electron configuration for neutral aluminum: [Ne] 3s2 3p1. This tells us aluminum has three valence electrons sitting in the third shell. When aluminum loses these three, it achieves the same stable arrangement as neon—a noble gas. But what happens if you try to remove a fourth electron?
To really see why the al most common ion charge is +3, let’s break down the actual ionization energies required to remove each electron from aluminum. Here’s what the data shows (source):
Notice the pattern? The energy jumps dramatically after the third electron. The first three electrons come from the outermost shell and are much easier to remove. But the fourth electron is part of an inner, tightly held shell that’s already stable—just like a noble gas. Trying to remove it would take almost five times more energy than the third!
This massive leap in required energy explains what charge is aluminum most likely to have. The atom simply won’t "pay" the energy cost to lose a fourth electron under normal chemical conditions. That’s why aluminum forms an ion with a charge of +3—it’s the sweet spot where the atom reaches stability with minimal energy input.
Another piece of the puzzle is the electron affinity of aluminum, which is the energy released when an atom gains an electron. For aluminum, this value is relatively low (42.5 kJ/mol), so the atom doesn’t have a strong tendency to gain extra electrons back after losing its three valence electrons (source). This reinforces why the +3 state is so stable and common.
So, how many electrons does al have after forming its most stable ion? Just 10—matching neon’s configuration. This is why the al most common ion charge you’ll encounter is always +3, and why aluminum is so reliable in its chemistry.
Understanding these energy jumps not only answers the question of what charge is aluminum but also sets the stage for how this charge influences aluminum’s behavior in real-world compounds. In the next section, we’ll compare this ionic charge with concepts like valency and oxidation state to clear up common student confusions.
Ever stared at a chemistry problem and wondered, “Wait—what’s the difference between ionic charge, valence, and oxidation state?” If you’re confused, you’re not alone! These terms sound similar but mean different things, especially when it comes to the charge of aluminum. Let’s break down each concept with simple explanations and a side-by-side comparison so you can confidently tackle any question about aluminum’s chemistry.
When you hear “ionic charge for aluminum,” think about the actual electrical charge an aluminum ion carries after it loses electrons. In most cases, aluminum forms a cation (Al3+) by shedding three electrons. This gives it a net charge of +3. The ion charge of aluminum is what you see in chemical formulas—like in Al2O3 oxidation, where each aluminum atom has a +3 charge.
Now, what about valence for aluminum? This is all about combining power. Valency tells you how many bonds an atom can form with other atoms. For aluminum, the valence number is also 3—because it can form three bonds by losing its three valence electrons. This value is especially important when predicting how aluminum will combine with other elements in compounds.
Finally, let’s talk about oxidation state. This term describes the hypothetical charge an atom would have if all bonds were completely ionic. In almost all aluminum compounds—including in al2o3 oxidation—aluminum’s oxidation state is +3. It’s a bookkeeping tool for tracking how electrons are distributed in a molecule or ion.
Let’s see these concepts side by side for clarity:
| Concept | Definition | Value for Aluminum |
|---|---|---|
| Ionic Charge | Net electric charge of the ion after electron transfer | +3 (Al3+) |
| Valency | Combining power; number of electrons lost, gained, or shared in bonding | 3 |
| Oxidation State | Hypothetical charge if all bonds were 100% ionic | +3 |
Imagine you’re looking at aluminum in Al2O3 (aluminum oxide):
For aluminum, the ionic charge, valency, and oxidation state are all +3—making it one of the most predictable metals in chemistry.
Why does this matter? Understanding these terms helps you read chemical formulas, balance equations, and predict how aluminum will react in both the lab and real-world applications. Now, as you move to the next section, you’ll see how this +3 charge shapes aluminum’s role in compounds you encounter every day—from protective oxides to essential industrial chemicals.
When you look at everyday materials—whether it’s the rust-resistant skin of an airplane or the antiperspirant in your bathroom—you’re seeing the charge of aluminum in action. But how does this +3 charge actually show up in the compounds we use and rely on? Let’s break down three of the most important aluminum compounds to see how the aluminum ion charge creates balanced, stable molecules.
Ever noticed how aluminum doesn’t rust like iron? That’s thanks to aluminum oxide (Al2O3). This compound forms a thin, tough layer on the surface of aluminum, protecting it from corrosion. Here’s the chemistry behind it:
To keep the compound electrically neutral, the charges must balance. Two aluminum ions (+6 total) pair with three oxide ions (−6 total):
The result? The formula for this compound is Al2O3. This is a classic example of how the charge of al shapes the formulas of its compounds. (See this balancing method in detail at Northwestern Polytechnic Chemistry Formulas.)
Let’s look at two more compounds where the +3 charge plays a starring role: aluminum chloride (AlCl3) and aluminum sulfate [Al2(SO4)3].
Here’s a quick summary table for clarity:
| Compound | Formula | Aluminum Ion Charge | Anion Charge | Net Charge |
|---|---|---|---|---|
| Aluminum Oxide | Al2O3 | +3 (Al3+) | −2 (O2−) | 0 (neutral) |
| Aluminum Chloride | AlCl3 | +3 (Al3+) | −1 (Cl−) | 0 (neutral) |
| Aluminum Sulfate | Al2(SO4)3 | +3 (Al3+) | −2 (SO42−) | 0 (neutral) |
Whenever you see aluminum in a compound, you can predict what is the charge for aluminum—it’s always +3. This predictability makes it easy to write and balance chemical equations, and to understand why the charge of al is so important in science and industry.
Next, let’s connect these chemical facts to the real world and see how aluminum’s +3 charge powers its role in industry, technology, and everyday life.
When you walk through a modern city, ride a train, or turn on your tap, you’re likely benefiting from the unique chemistry of aluminum. But what makes this metal so valuable in industry? The answer traces back to the charge of aluminum—specifically, its +3 ionic charge. Let’s explore how this simple atomic property drives aluminum’s role in metallurgy, water purification, and construction, and why it’s at the heart of high-quality manufacturing worldwide.
First, let’s tackle a common question: is al a metal? Absolutely! Aluminum is a lightweight, silvery-white metal with remarkable versatility. Its metallic nature is closely tied to its ability to form a stable Al3+ ion. When aluminum is refined and used in alloys, this +3 charge enables it to bond effectively with other elements, resulting in materials that are both strong and corrosion-resistant.
Imagine an airplane wing or a skyscraper frame: these structures rely on aluminum alloys that combine lightness with durability. The +3 charge is what makes these alloys possible, giving engineers the flexibility to design safer, longer-lasting products.
Ever wondered how drinking water is made crystal clear? Here’s where aluminum’s charge steps into another vital role. Aluminum sulfate, a compound built from Al3+ ions, is widely used in water treatment plants as a coagulant. When added to water, it causes tiny suspended particles to clump together—a process called flocculation—so they can be easily removed (source).
So, the next time you fill a glass with clean water, remember: it’s the powerful +3 charge of aluminum working behind the scenes to keep it safe and clear.
Now, let’s bring it all together in the world of construction. Is aluminium a metal that’s up to the challenges of modern building? Absolutely—and here’s why:
For manufacturers like Shengxin, understanding and leveraging the charge of aluminum is essential. It’s not just about chemistry in a textbook—it’s about producing high-quality, durable aluminum profiles that stand up to real-world demands. The formation of alumina, the ability to alloy, and the use in water treatment all stem from this fundamental +3 charge.
The +3 charge of aluminum is more than a scientific detail—it’s the engine behind its corrosion resistance, versatility in alloys, and critical role in water purification and construction.
As industries continue to innovate, the reliable charge of aluminum ensures that products—from aircraft and skyscrapers to clean water—are built on a foundation of chemical stability and performance. This deep connection between atomic structure and industrial application is what makes aluminum one of the world’s most indispensable metals.
Aluminum ions have a charge of +3 because a neutral aluminum atom loses its three valence electrons during chemical reactions, resulting in a stable Al3+ ion. This electron loss is energetically favorable, making +3 the only common ionic charge for aluminum in compounds.
Aluminum's atomic structure features 13 protons and 13 electrons in a neutral atom, with three electrons in its outermost shell. These three valence electrons are easily lost, leading to a consistent +3 charge when aluminum becomes an ion.
Removing more than three electrons from aluminum requires a significant increase in ionization energy, making it highly unfavorable. After losing three valence electrons, aluminum achieves a stable noble gas electron configuration, so it does not naturally form +1 or +2 ions.
The +3 charge enables aluminum to form strong, stable compounds and a protective oxide layer that resists corrosion. This property is essential for manufacturing durable aluminum profiles used in construction, transportation, and water purification.
Common aluminum-containing compounds include aluminum oxide (Al2O3), aluminum chloride (AlCl3), and aluminum sulfate [Al2(SO4)3]. In each, aluminum ions carry a +3 charge, balancing the negative charges of the corresponding anions.
 บริการออนไลน์
บริการออนไลน์ 0086 136 3563 2360
0086 136 3563 2360 sales@sxalu.com
sales@sxalu.com +86 136 3563 2360
+86 136 3563 2360